Describe the Four Basic Steps of the Fractional Distillation Process
Heating is usually done with high pressure steam to temperatures of about 1112 degrees Fahrenheit 600 degrees Celsius. The design of fractionation columns is normally made in two steps.

Fractional Distillation Definition Application And Examples
5 Steps of Fractional Distillation.

. The main difference between fractional distillation and simple distillation is that simple distillation separate liquids with boiling point gaps of at least 50 degrees whereas fractional distillation separates liquids with closer boiling points. What is Fractional Distillation. 3 after vaporization components begin to separate.
The technique is used in labs and in industry where the. Few fractional distillation apparatuses are required for the process. The crude oil is heated to high temperatures and evaporates.
1- heat mixture to begin boiling. Crude oil is heated until it evaporates. Fractional distillation includes the following steps.
Fractional distillation of crude oil. Fractional distillation is a process by which components in a chemical mixture are separated into different parts called fractions according to their different boiling points. Simple distillation involves heating the liquid mixture to the boiling point.
The main difference is that all the liquid condensate is returned to the upstream still. Advantages of Fractional Distillation Process. Due to the large number of stages involved and also due to reflux the product purity obtained is also good.
It is a process of separation of a chemical mixture whose components have different boiling points. 2 vapor appears as mixture comes to boil. 2 - vapor appears as mixture comes to boil.
Commonly used with Bourdon gauges. Automation Controls Engineering. A greater degree of separation between products is.
5 cool down causes condensation and liquid is separately collected. 3 - after vaporization components begin to separate. Even substances which have extremely comparable boiling temperatures can be accurately separated by fractional distillation.
Concept of Process System Design. Boiling temperatures are what enable fractional distillation to be each relatively easy and affordable. 4 as it rises vapor eventually cools.
As the feed is partially vaporised in the first still the vapors rise travel through the. Droplets of liquid should be seen in the fractional column but there should never be a large pool of liquid flooding. Naturally to boil a substance its essential to heat it.
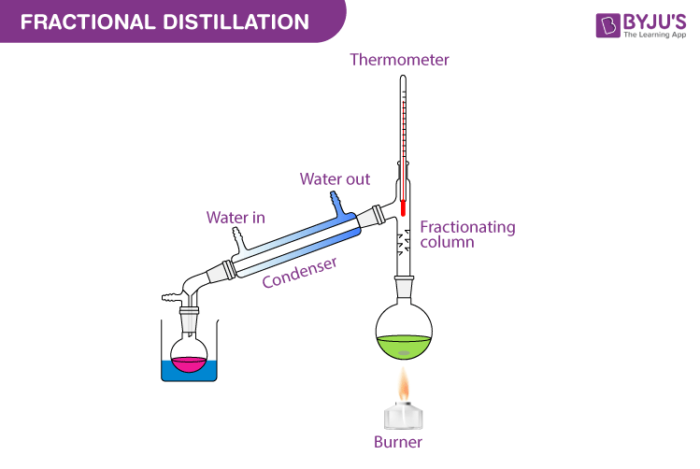
Fractional distillation separates hydrocarbons using their different boiling points. It includes distilling flask condenser receiver fractionating column thermometer and heat source. This article looks at 1.
Place a mixture in the fractional distillation column and add heat until the temperature reaches the boiling point of the chemical compound within the substance with the lowest boiling point. Preset reducing valve - is used only to give out the amount of pressure set by manifold. It is useful for separating ethanol from a mixture of ethanol and water and for separating crude oil into.
After setting up the apparatus a mixture of two miscible liquids A and B is taken where A has more volatility than substance B. Separates miscible liquids that have different boiling points. The steps of the process are.
You heat the mixture of two or more substances liquids with different boiling points to a high temperature. A tall fractionating column is fitted above the mixture with. In this process the the mixture is boiled in a round.
Crude oil vapour is put into a fractionating column at the bottom and rises upwards. Similar to continuous shell still the fractional distillation process is made up of several stills linked together in series. Adjustable reducing valve - is used on the high pressure system to.
The evaporated mixture rises up the fractionating column which has a temperature gradient. 5 Steps of Fractional Distillation. Process Mixture Characteristics.
It is a steady state continuous process hence a large amount of feed can be processed and a large amount of products can be obtained. After we set up the apparatus the mixture of liquids A and B which are miscible are taken where A has greater volatility than B substance. Adjustable reducing valve - is used on the high pressure system to the regulator from high to low pressure to the system.
Some of the fractional distillation apparatuses in use for the process are distilling flask receiver thermometer heat source condenser and fractionating column. Preset reducing valve - is used only to give out the amount of pressure set by manifold. Fractional distillation is used to purify chemicals and to separate mixtures to obtain their components.
Fractional distillation separates a mixture into a number of different parts called fractions. Preset reducing valve adjustable reducing valve and multiple stage reducing valve. Step-by-Step Procedures for Fractional Distillation is shared under a CC BY-NC-ND 40 license and was authored.
1- heat mixture to begin boiling. A process design followed by a mechanical design. Preset reducing valve adjustable reducing valve and multiple stage reducing valve.
The purpose of the process design is to calculate the number of required theoretical stages and stream flows including the reflux ratio heat reflux and other heat duties. It is an special type of distillation in which two miscible liquids having different boiling points but closed to each other are separated by fractionating column. The Procedure of Fractional Distillation.
The basic process of fractional distillation involves the following steps. The steps of fractional distillation are as follows. If the column floods allow the liquid to drain back into the distilling flask and heat at a gentler rate.

What Is Fractional Distillation Definition Process Video Lesson Transcript Study Com

Basic Steps To Fractional Distillation Understanding Distillation
Fractional Distillation Detailed Explanation Along With Diagrams

Crude Oil Fractional Distillation 4 2 1 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams
Comments
Post a Comment